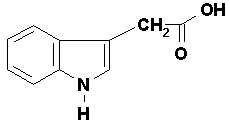
|
History of Auxins and Pioneering
Experiments
Auxins were the first plant hormones discovered.
Charles Darwin was among the first scientists to dabble in plant
hormone research. In his book "The Power of Movement in Plants"
presented in 1880, he first describes the effects of light on movement
of canary grass (Phalaris canariensis) coleoptiles. The coleoptile
is a specialized leaf originating from the first node which sheaths
the epicotyl in the plants seedling stage protecting it until it
emerges from the ground. When unidirectional light shines on the
coleoptile, it bends in the direction of the light. If the tip of
the coleoptile was covered with aluminum foil, no bending would
occur towards the unidirectional light. However if the tip of the
coleoptile was left uncovered but the portion just below the tip
was covered, exposure to unidirectional light resulted in curvature
toward the light. Darwin's experiment suggested that the tip of
the coleoptile was the tissue responsible for perceiving the light
and producing some signal which was transported to the lower part
of the coleoptile where the physiological response of bending occurred.
He then cut off the tip of the coleoptile and exposed the rest of
the coleoptile to unidirectional light to see if curving occurred.
Curvature did not occur confirming the results of his first experiment
(Darwin, 1880).
It was in 1885 that Salkowski discovered indole-3-acetic acid (IAA)
in fermentation media (Salkowski, 1885). The isolation of the same
product from plant tissues would not be found in plant tissues for
almost 50 years. IAA is the major auxin involved in many of the
physiological processes in plants (Arteca, 1996). In 1907, Fitting
studied the effect of making incisions on either the light or dark
side of the plant. His results were aimed at understanding if translocation
of the signal occurred on a particular side of the plant but his
results were inconclusive because the signal was capable of crossing
or going around the incision (Fitting, 1907). In 1913, Boysen-Jensen
modified Fritting's experiment by inserting pieces of mica to block
the transport of the signal and showed that transport of auxin toward
the base occurs on the dark side of the plant as opposed to the
side exposed to the unidirectional light (Boysen-Jensen, 1913).
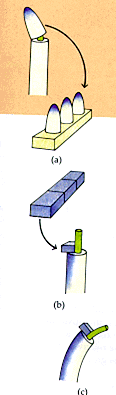
In 1918, Paal confirmed Boysen-Jensen's results by cutting off coleoptile
tips in the dark, exposing only the tips to the light, replacing
the coleoptile tips on the plant but off centered to one side or
the other. Results showed that whichever side was exposed to the
coleoptile, curvature occurred toward the other side (Paal, 1918).
Soding was the next scientist to extend auxin research by extending
on Paal's idea. He showed that if tips were cut off there was a
reduction in growth but if they were cut off and then replaced growth
continued to occur (Soding, 1925).
In 1926, a graduate student from Holland by the name of Fritz Went
published a report describing how he isolated a plant growth substance
by placing agar blocks under coleoptile tips for a period of time
then removing them and placing them on decapitated Avena stems (Went,
1926). After placement of the agar, the stems resumed growth (see
below). In 1928, Went developed a method of quantifying this plant
growth substance. His results suggested that the curvatures of stems
were proportional to the amount of growth substance in the agar
(Went, 1928). This test was called the avena curvature test.(see
below) 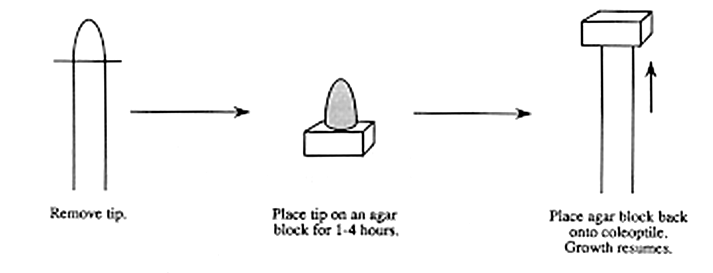
 Much
of our current knowledge of auxin was obtained from its applications.
Went's work had a great influence in stimulating plant growth substance
research. He is often credited with dubbing the term auxin but it
was actually Kogl and Haagen-Smit who purified the compound auxentriolic
acid (auxin A) from human urine in 1931 (Kogl and Haagen-Smit, 1931).
Later Kogl isolated other compounds from urine which were similar
in structure and function to auxin A, one of which was indole-3
acetic acid (IAA) initially discovered by Salkowski in 1985. In
1954 a committee of plant physiologists was set up to characterize
the group auxins. The term comes from the Greek auxein meaning "to
grow." Compounds are generally considered auxins if they are
synthesized by the plant and are substances which share similar
activity to IAA (the first auxin to be isolated from plants) (Arteca,
1996; Davies, 1995). Much
of our current knowledge of auxin was obtained from its applications.
Went's work had a great influence in stimulating plant growth substance
research. He is often credited with dubbing the term auxin but it
was actually Kogl and Haagen-Smit who purified the compound auxentriolic
acid (auxin A) from human urine in 1931 (Kogl and Haagen-Smit, 1931).
Later Kogl isolated other compounds from urine which were similar
in structure and function to auxin A, one of which was indole-3
acetic acid (IAA) initially discovered by Salkowski in 1985. In
1954 a committee of plant physiologists was set up to characterize
the group auxins. The term comes from the Greek auxein meaning "to
grow." Compounds are generally considered auxins if they are
synthesized by the plant and are substances which share similar
activity to IAA (the first auxin to be isolated from plants) (Arteca,
1996; Davies, 1995).
Biosynthesis and Metabolism of Auxin
IAA is chemically similar to the amino acid
tryptophan which is generally accepted to be the molecule from which
IAA is derived. Three mechanisms have been suggested to explain
this conversion:
Tryptophan is converted to indolepyruvic acid through a transamination
reaction. Indolepyruvic acid is then converted to indoleacetaldehyde
by a decarboxylation reaction. The final step involves oxidation
of indoleacetaldehyde resulting in indoleacetic acid.
Tryptophan undergoes decarboxylation resulting in tryptamine. Tryptamine
is then oxidized and deaminated to produce indoleacetaldehyde. This
molecule is further oxidized to produce indoleacetic acid.
As recently as 1991, this 3rd mechanism has evolved. IAA can be
produced via a tryptophan-independent mechanism. This mechanism
is poorly understood, but has been proven using trp(-) mutants.
Other experiments have shown that, in some plants, this mechanism
is actually the preferred mechanism of IAA biosynthesis.
The enzymes responsible for the biosynthesis of IAA are most active
in young tissues such as shoot apical meristems and growing leaves
and fruits. The same tissues are the locations where the highest
concentrations of IAA are found. One way plants can control the
amount of IAA present in tissues at a particular time is by controlling
the biosynthesis of the hormone. Another control mechanism involves
the production of conjugates which are, in simple terms, molecules
which resemble the hormone but are inactive. The formation of conjugates
may be a mechanism of storing and transporting the active hormone.
Conjugates can be formed from IAA via hydrolase enzymes. Conjugates
can be rapidly activated by environmental stimuli signaling a quick
hormonal response. Degradation of auxin is the final method of controlling
auxin levels. This process also has two proposed mechanisms outlined
below:
The oxidation of IAA by oxygen resulting in the loss of the carboxyl
group and 3-methyleneoxindole as the major breakdown product. IAA
oxidase is the enzyme which catalyzes this activity. Conjugates
of IAA and synthetic auxins such as 2,4-D can not be destroyed by
this activity.
C-2 of the heterocyclic ring may be oxidized resulting in oxindole-3-acetic
acid. C-3 may be oxidized in addition to C-2 resulting in dioxindole-3-acetic
acid.
The mechanisms by which biosynthesis and degradation of auxin molecules
occur are important to future agricultural applications. Information
regarding auxin metabolism will most likely lead to genetic and
chemical manipulation of endogenous hormone levels resulting in
desirable growth and differentiation of important crop species.
Ultimately, the possibility exists to regulate plant growth without
the use of hazardous herbicides and fertilizers (Davies, 1995; Salisbury
and Ross, 1992).
Functions of Auxin
The following are some of the responses that
auxin is known to cause (Davies, 1995; Mauseth, 1991; Raven, 1992;
Salisbury and Ross, 1992).
- Stimulates cell elongation
- Stimulates cell division
in the cambium and, in combination with cytokinins in tissue culture
- Stimulates differentiation
of phloem and xylem
- Stimulates root initiation
on stem cuttings and lateral root development in tissue culture
- Mediates the tropistic
response of bending in response to gravity and light
- The auxin supply from
the apical bud suppresses growth of lateral buds
- Delays leaf senescence
- Can inhibit or promote
(via ethylene stimulation) leaf and fruit abscission
- Can induce fruit setting
and growth in some plants
- Involved in assimilate
movement toward auxin possibly by an effect on phloem transport
- Delays fruit ripening
- Promotes flowering
in Bromeliads
- Stimulates growth
of flower parts
- Promotes (via ethylene
production) femaleness in dioecious flowers
- Stimulates the production
of ethylene at high concentrations
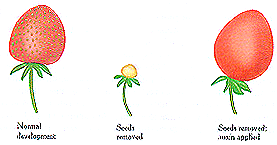
Above describes the effect of auxin on strawberry development. The
achenes produce auxin. When removed the strawberry does not develop
(Raven, 1992). |